Virus Science Case
Virus Science Case
Re: the Legality of Health Acts supported by Science that does not follow the Scientific Method
Facts of the Case
- Dates: Oct 2022
- Location: Hamburg, Germany
- Court: Hamburg District Court
- Case #:
- Plaintiff: Marvin Haberland
- Plaintiff’s Lawyer:
- Defendant: City of Hamburg
- Trial Type:
- Judge:
- Status: Ongoing
- Verdict: TBD
*updated Oct 16, 2022
Background
A German engineer and researcher Marvin Haberland has challenged the court for a fine he received for not wearing a mask. As he describes in an online interview with New Zealand Dr Sam Bailey, he is taking this as an opportunity to challenge the legality of the German covid measures. These measures he says are based on invalid scientific methodologies which the German law says should be illegal. [1]
He states that if he were to directly challenge the law, the German courts would not allow his case to be heard. However a case challenging a fine would be heard and offers an opportunity to challenge the health law and the science of virology. [1]
A court date was set for October 19 at the Hamburg District Court. It was awaited with great excitement – and many people had also already announced their intention to attend the public hearing. So this trial is not only unique, but also explosive. [2]
But now the district court has canceled the date. The reason given is that the judge has fallen ill. The court has not yet announced when the trial, scheduled for October 19, will be rescheduled. [2]
Regarding the merits of his case, Haberland said
“If the judge should actually rule against me, then that would be okay for me personally in a way. Because then we would have a verdict that says: In virology, you don’t have to do any control experiments, so to speak. But that would, to a certain extent, make the whole thing ridiculous. [3]
In any case, the judge has everything before him – and he is actually completely free in his decision. Either he examines all my evidence requests and then makes his judgment or he does not look at all this and rejects the evidence requests. Then, however, he would have to justify why he does that. After that, I would go to the next court instance. And there is no “alternative” anymore, i.e. everything has to be examined. In this respect, nothing bad can actually happen now. One way or another, it will lead to success in terms of educational work.” [3]
Significance
If this case is upheld it would show that the science of virology has not followed the scientific measures and all measures and restrictive acts would have to end.
Plaintiff’s Argument
Essentially the plaintiff argues that the German Law and the Infection Protection Act, requires all science to follow the “Scientific Method”. This would entail controlled studies and detailed recording of methodologies to show that whatever is claimed could not be falsified. Mr Haberland argues that this required process was never completed and that consequently none of the covid measures are legal. [1]
In a German interview with Transition News:
“I have been working intensively on the subject of health, nutrition and medicine for five years now and have come across the fact that there is quite a controversial discussion in science as to whether things are done scientifically in virology and whether the scientific method is applied correctly. After all, even in the German Infection Protection Act, which is quite unique in the world, it is explicitly stated right at the beginning in paragraph 1 that everyone involved, every researcher, every institution, every authority, every university and ultimately also every doctor is obliged to work according to the state of the art in science. [3]
In particular, this also includes conducting control experiments. That is quite clear. Therefore, I have meticulously reviewed all relevant publications and asked the Robert Koch Institute and all authorities for publications in which the pathogen has been detected. You, Torsten, had also written to five authors who have carried out authoritative studies in the matter of SARS-CoV-2 detection – and I have also read all these studies. I noticed the same thing that Stefan Lanka and other scientists have found out, namely that in none of these studies corresponding control experiments were made.” [3]
A control experiment he explains is:
“For example, I want to prove that I can break a window pane with a feather. To do this, I have to do an experiment that can prove or verify this hypothesis – and then I also have to offer an experiment that can be used to falsify the hypothesis. [3]
For this I tie the feather to a stone so that it can fly better. Afterwards I throw the stone and the feather in connected form through the window and see what happens. Of course, the window also breaks. [3]
If I did not do a control experiment, I could theoretically conclude that it was the spring that caused the pane to break. The control experiment would now consist of taking away the independent variable to be investigated, i.e. the spring, and throwing the stone alone through the window – and then seeing whether the glass then remains intact or not. Only then will I know for sure whether the spring can be held causally responsible for the glass breakage. By the way, the spring stands here for the particles, which are claimed to be viruses. But the spring cannot be held accountable for the shattering of the window, because of course this also happens when the stone hits the glass alone. [3]
Such an experiment has really never been done. And there are even institutions that openly admit that.” [3]
“As part of my court case, I sent a request based on the Freedom of Information Act to the Doherty Intsitute at the University of Melbourne. This institute had published a study in 2020 that was the first outside of China that virologists claimed to have isolated “SARS-CoV-2.” I wanted to know from the authors of this paper whether they had carried out corresponding control experiments. And they actually wrote me back that they had not done such control experiments. [3]
Thereupon I asked: Why don’t you do any controls? And the answer was that they didn’t have enough capacity left for that. Their study was published as having no control experiment. But also in the guidelines of the German Research Foundation, for example, it is clearly stated with regard to what scientific work means that one should not publish early before one has finished all documentation and thus also all control experiments. [3]
I also received an answer from France, namely from the scientific director of the world-famous Institut Pasteur in Paris, which corresponds to the Robert Koch Institute here. According to this, no control tests were carried out there either. This, too, is now before the judge. He must now decide whether, according to Section 1 of the Infection Protection Act, the scientific character is still given.” [3]
“It is completely undisputed in science that you have to control your results. That is also recognized worldwide. The Charité itself also writes that in its guidelines. Just like the Robert Koch Institute, they are committed to the methods of good scientific practice published by the German Research Foundation. And every researcher has these guidelines in his or her employment contract. Among other things, it says that you should always and consistently challenge yourself, that you should document everything you do. And everyone who receives research funding in Germany is obliged to comply with these scientific rules. In this respect, this also applies to the Charité. [3]
I have submitted numerous motions for evidence. These include a request to the judge that he ask a biologist of his choice whether he can present a study in which a virus has been detected, including a control experiment. Another request to the court is to ask a laboratory or virologist to perform an appropriate experiment.” [3]
“In the soldiers’ trial before the Federal Administrative Court, for example, extensive motions for evidence were filed and experts were even heard – and yet in the end, this highest German court did not appreciate all this and ruled against the plaintiff soldiers. Does something like that affect your confidence? [3]
Of course, you can never say that there are no viruses. Because you can’t prove the non-existence of something. You can only prove the existence of something. And, it should be emphasized again, at least according to the current state of science and according to the scientific methods, no one has ever proven a virus.” [3]
Defendant’s Argument
…More information is needed…
Relevant Prior Judgements/ Cases
…More information is needed…
Decision
…More information is needed…
Aftermath
…More information is needed…
Further Research
Court Documents:
- …More information is needed…
Supporting Documents:
- Corona Fakten Telegram Channel
- The “Settling The Virus Debate” Statement
- Christine Massey FOIA
- Dr. Sam Bailey – Secrets of Virology “Control” Experiments
- Dr. Sam Bailey – The Truth About Virus Isolation
- Spanish Flu Video – Secrets of Influenza
In the news:
- …More information is needed…
Media
References
Keyword
Bailey, Charité, Control, Controlled Experiment, Doherty Intsitute, Engelbrecht, Existence, FOIA, German Infection Protection Act, Germany, Haberland, Hamburg, Isolation, Lanka, Mania, Marvin, Massey, RKI, Robert Koch Institute, Science, Scientific Method, University of Melbourne, Virology, Virus
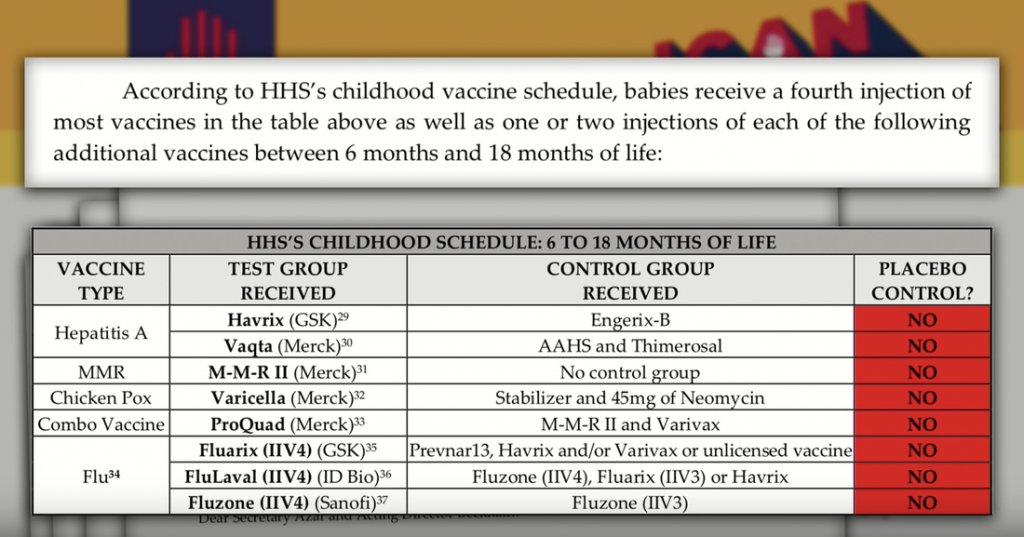
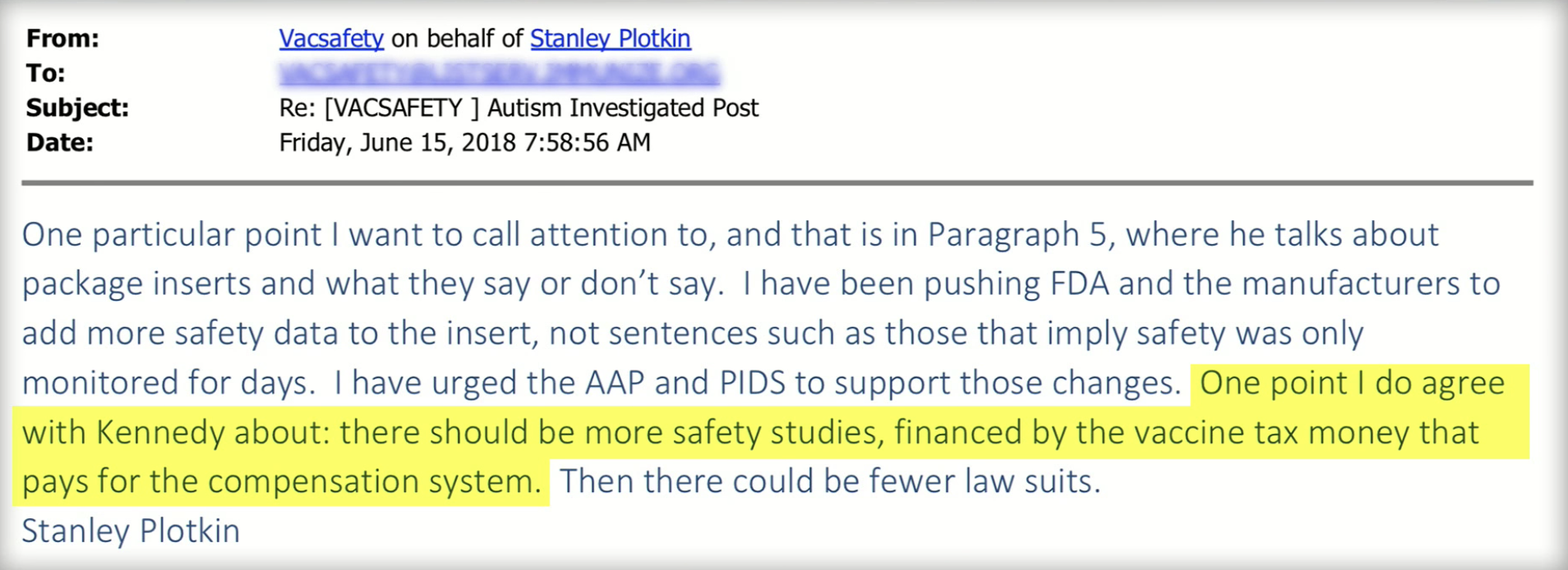
![[SOURCE] Page 14 ICAN Response December 31, 2018](http://coronacases.wiki/wp-content/uploads/2022/04/screen-shot-2019-01-27-at-3-44-49-pm_orig-1024x352.png)