GSK Safety Fraud Case
GSK Safety Fraud Case
Re: the Legality of DeFrauding the Public with False Safety Data , Bribery & False Promotion of Several Drugs
Facts of the Case
aka: U.S. v. GlaxoSmithKline LLC
- Dates: July 2, 2012
- Location: Massachusetts, USA
- Court: U.S. District Court for the District of Massachusetts
- Case #: 12-cr-10206
- Plaintiff: U.S. Attorney’s Office for the District of Massachusetts
- Plaintiff’s Lawyer:
- Defendant: GlaxoSmithKline LLC (GSK)
- Trial Type:
- Judge:
- Status: Decided (July 2, 2012)
- Verdict: for the Plaintiff
*updated Nov 12, 2022
Background
the British drugmaker was accused of breaking U.S. laws in the marketing and development of pharmaceuticals. [3]
including illegally promoting the antidepressants in the U.S. for uses that weren’t approved by the Food and Drug Administration, a practice known as off-label marketing, and withholding important safety data about Avandia from the U.S. regulator.[5]
The government’s case against Glaxo was based partly on a lawsuit filed by former Glaxo employees (whistle-blowers) in federal court in Boston in 2003. Thomas Gerahty, a former senior marketing development manager, and Matthew Burke, a former regional vice president, filed a so-called qui tam suit against Glaxo on the government’s behalf under the Federal False Claims Act, which prohibits people or businesses from defrauding the government, and provides incentives for those who suspect wrongdoing to come forward. [5]
According to their lawyer, Erika Kelton, Messrs. Gerahty and Burke provided information to the government about Glaxo’s marketing practices, including the use of off-label promotion and financial inducements to doctors to prescribe Glaxo’s drugs. Others, including former Glaxo sales manager Lois Graydon, also filed lawsuits against the company, according to Ms. Graydon’s lawyers. [5]
The whistle-blowers collectively stand to receive millions of dollars as a share of the government’s settlement, under the terms of the False Claims Act. Ms. Kelton said the exact amount hasn’t been determined yet, but plaintiffs generally are entitled to receive 15% to 30% of government recoveries in civil cases. [5]
Crimes and civil violations like those in the GlaxoSmithKline case have been widespread in the pharmaceutical industry and have produced a series of case with hefty fines. One reason some have said the industry regards the fines as simply a cost of doing business is because aggressively promoting drugs to doctors for uses not officially approved — including inducing other doctors to praise the drugs to colleagues at meetings — has quickly turned numerous drugs from mediocre sellers into blockbusters, with more than $1 billion in annual sales. [4]
In the last few years, the Justice Department has become much more aggressive in pursuing such fraud, often in whistleblower cases taken on by a handful of U.S. attorneys focused on such fraud. Among the most active (in 2012) are the U.S. attorneys in Boston, Philadelphia and San Francisco — all in regions with numerous pharmaceutical and biotech company operations. [4]
GSK targeted the antidepressant Paxil to patients under age 18 when it was approved for adults only, and it pushed the drug Wellbutrin for uses it was not approved for, including weight loss and treatment of sexual dysfunction, according to an investigation led by the U.S. Justice Department. [3]
The company went to extreme lengths to promote the drugs, such as distributing a misleading medical journal article and providing doctors with meals and spa treatments that amounted to illegal kickbacks, prosecutors said. [3]
In a third instance, GSK failed to give the U.S. Food and Drug Administration safety data about its diabetes drug Avandia, in violation of U.S. law, prosecutors said. [3]
The misconduct continued for years beginning in the late 1990s and continued, in the case of Avandia’s safety data, through 2007. [3]
Over a period of more than a decade, the government’s latest investigation found, the company plied doctors with perks such as free spa treatments, Colorado ski trips, pheasant-hunting jaunts to Europe and Madonna concert tickets, Justice Department officials said. [5]
Meanwhile, it encouraged those doctors to write prescriptions for the two antidepressants that went beyond their sanctioned uses. U.S. law bars companies from marketing a drug for uses not approved by the Food and Drug Administration, though doctors may prescribe approved drugs as they see fit. [5]
The criminal case is being prosecuted by the U.S. Attorney’s Office for the District of Massachusetts and the Civil Division’s Consumer Protection Branch. The civil settlement was negotiated by the U.S. Attorney’s Office for the District of Massachusetts, the U.S. Attorney’s Office for the District of Colorado and the Civil Division’s Commercial Litigation Branch. Assistance was provided by the HHS Office of Counsel to the Inspector General, Office of the General Counsel-CMS Division and FDA’s Office of Chief Counsel as well as the National Association of Medicaid Fraud Control Units. [1]
This matter was investigated by agents from the HHS-OIG; the FDA’s Office of Criminal Investigations; the Defense Criminal Investigative Service of the Department of Defense; the Office of the Inspector General for the Office of Personnel Management; the Department of Veterans Affairs; the Department of Labor; TRICARE Program Integrity; the Office of Inspector General for the U.S. Postal Service and the FBI. [1]
This resolution is part of the government’s emphasis on combating health care fraud and another step for the Health Care Fraud Prevention and Enforcement Action Team (HEAT) initiative, which was announced in May 2009 by Attorney General Eric Holder and Kathleen Sebelius, Secretary of HHS. The partnership between the two departments has focused efforts to reduce and prevent Medicare and Medicaid financial fraud through enhanced cooperation. Over the last three years, the department has recovered a total of more than $10.2 billion in settlements, judgments, fines, restitution, and forfeiture in health care fraud matters pursued under the False Claims Act and the Food, Drug and Cosmetic Act. [1]
GlaxoSmithKline Company History
GlaxoSmithKline (GSK) started in 1715 as Plough Court pharmacy, a small apothecary shop in London. Through multiple corporate mergers, GSK found its current form in 2001. It manufactures prescription medicines, vaccines and consumer health products. [6]
GlaxoSmithKline is a Big Pharma corporation. (as of March 2021) It sits at No. 282 on Forbes’ Global 500 rankings. GSK is ranked number 6 among the top 20 pharma companies by revenue in 2019, according to FiercePharma. [6]
The British pharmaceutical company is well-known for products like Advair, Ventolin, Theraflu, Advil and Centrum. [6]
Total revenues for GSK reached $34 billion pounds in 2020. This equates to $47.3 billion in U.S. currency. [6]
In 2020, GSK employed about 94,000 people in over 150 countries. Its U.S. headquarters are in Philadelphia. GSK has a separate research facility in North Carolina. [6]
GSK focuses on vaccines, pharmaceutical drugs and consumer health care products. The company’s vaccine business distributes about 2 million vaccines a day to around 160 countries. [6]
GSK is the maker of the industry’s broadest range of inhaled respiratory products. These include Advair, Ventolin and Breo Ellipta. The company has also partnered with Pfizer and Shionogi to form a global HIV business, ViiV Healthcare. [6]
GSK’s respiratory and HIV pharmaceutical businesses are its top moneymakers. Overall, its pharmaceuticals business raked in about $24 billion (17 million pounds) in 2020. [6]
But GSK has faced allegations of unethical and unsafe business practices. Some of its former employees were criminally prosecuted on two different continents. [6]
Some of GSK’s popular pharmaceuticals have led to product liability lawsuits. These include Avandia, Zantac, Paxil, Prevacid 24HR, Nexium 24HR and Zofran. [6]
Significance
The resolution is the largest health care fraud settlement in U.S. history and the largest payment ever by a drug company. [1]
Under the provisions of the Food, Drug and Cosmetic Act, a company in its application to the FDA must specify each intended use of a drug. After the FDA approves the product as safe and effective for a specified use, a company’s promotional activities must be limited to the intended uses that FDA approved. In fact, promotion by the manufacturer for other uses – known as “off-label uses” – renders the product “misbranded.” [1]
Guilty pleas in cases of alleged corporate misconduct are exceedingly rare, making GSK’s agreement especially unusual. [3]
The GSK settlement surpasses what had been the largest criminal case involving a drugmaker in U.S. history. In 2009, Pfizer Inc agreed to pay $2.3 billion to settle allegations it improperly marketed 13 drugs. [3]
Plaintiff’s Argument
Much of the misconduct alleged by the government occurred in the 1990s and early 2000s, a period of booming drug sales and widespread illegal marketing among big drug makers, many of whom have since paid large sums to the government to settle criminal and civil investigations stemming from those practices. With many companies promoting drugs of similar effectiveness, as was true with antidepressants and other mass-market pills,aggressive marketing became the key to driving sales. [5]
off-label marketing
It is illegal to promote uses for a drug that have not been approved by the Food and Drug Administration — a practice known as off-label marketing. [4]
- Paxil
Prosecutors said GlaxoSmithKline illegally promoted the drug Paxil for treating depression in children from April 1998 to August 2003, even though the FDA never approved it for anyone under age 18. T [4]
the government said Glaxo spent six years—1998 to 2003—unlawfully promoting Paxil for patients under 18 when the drug wasn’t approved by the FDA for non-adults.
- Wellbutrin
The corporation also promoted the drug Wellbutrin from January 1999 to December 2003 for weight loss, the treatment of sexual dysfunction, substance addictions and attention deficit hyperactivity disorder, although it was only approved for treatment of major depressive disorder.
The government also said Glaxo illegally promoted Wellbutrin—approved solely to treat depression—for a number of other purposes, including weight loss, treatment of sexual dysfunction, substance addiction and attention deficit hyperactivity disorder. [5]
According to the government, “Glaxo sales representatives sometimes referred to Wellbutrin as ‘the happy, horny, skinny pill’ as a way to remind doctors of the unapproved uses.” [5]
Safety Data for Avandia
Justice Department officials also said that between 2001 and 2007 GlaxoSmithKline failed to report to the FDA on safety data from certain post-marketing studies and from two studies of the cardiovascular safety of the diabetes drug Avandia. Since 2007, the FDA has added warnings to the Avandia label to alert doctors about potential increased risk of congestive heart failure and heart attack. [4]
The Justice Department also said that between 2001 and 2007, Glaxo failed to report certain safety data to the FDA about Avandia, formerly one of the top-selling diabetes treatments in the world. The missing data included two studies that examined the cardiovascular safety of Avandia. Avandia now carries black-box warnings about heart risk. The FDA put tight curbs on its use in 2010, while European regulators ordered it withdrawn from the market. [5]
Bribery
“GSK’s sales force bribed physicians to prescribe GSK products using every imaginable form of high priced entertainment, from Hawaiian vacations to paying doctors millions of dollars to go on speaking tours to a European pheasant hunt to tickets to Madonna concerts, and this is just to name a few,” said Carmin M. Ortiz, U.S. attorney in Massachusetts. [4]
False Article
It said Glaxo helped prepare an article published in a medical journal in 2001 that falsely reported Paxil had proven effective at treating depression in children in a clinical trial, when the trial showed no such thing. [5]
Glaxo sales representatives then used the article to promote the drug’s use in depressed adolescents, the government said. The clinical trial, called Study 329, has been widely criticized by the medical community in the years since its publication. [5]
The government said Glaxo paid an outside medical-publications company to ghost-write the article about Study 329. The article became a centerpiece of Senate investigations into drug companies’ practice of hiring ghost-writers to draw up articles tlater published under the names of academic authors. [5]
Defendant’s Argument
…More information is needed…
Relevant Prior Judgements/ Cases
Prior GSK Lawsuits & Fines
- In 2011, GSK agreed to pay nearly $41 million to 37 states and the District of Columbia in an unrelated case about substandard manufacturing processes at a Puerto Rico factory. [3]
- In 2010, the company took a $2.4 billion charge in connection with Avandia to settle claims from patients. [3]
- GSK reported that it had been named in about 1,200 Zantac lawsuits on its 2020 annual report. In December 2020, the court granted GSK’s motion to dismiss claims on innovator liability. As of March 2021, there have been no verdicts or settlements. [6]
- GSK faced 476 federal Zofran lawsuits in February 2021. The first bellwether trial has been set for Oct. 18, 2021. [6]
- More than 13,700 PPI lawsuits are pending in federal court in New Jersey. GSK is named in thousands of claims for Prevacid 24HR and Nexium 24HR, the company said in its 2020 annual report. The first PPI bellwether is set for November 2021. [6]
- In March 2020, the Department of Justice (DOJ) sent GSK a notice of a civil investigation it had opened into allegations of False Claims Act violations by the company because of Zantac. DOJ requested documents on June 18, 2020. On the same day, the New Mexico Attorney General filed a lawsuit against GSK for violations of false advertising statutes and state consumer protection, among other claims. [6]
- GlaxoSmithKline recalled some of its products for defects, possible tampering and reduced effectiveness. Products affected include consumer weight loss products, vaccines and albuterol inhalers. [6]
- A Chinese court ordered GlaxoSmithKline to pay $492 million in 2014. The fine resolved charges of bribing doctors in China to use GSK products. It was the biggest penalty ever imposed by a Chinese court. [6]
The court sentenced Briton Mark Reilly to four years in prison. He was the company’s British executive for China. Four other Chinese employees received the same punishment. [6]
But the employees may never serve prison time. The court postponed the sentences. Reilly was deported. [6]
Prior Lawsuits & Fines by other Pharmaceuticals
- The prior record-setting case involved Pfizer Inc., the world’s biggest drugmaker. Pfizer paid the government $2.3 billion in criminal and civil fines for improperly marketing 13 different drugs, including Viagra and cholesterol fighter Lipitor. Pfizer was accused of encouraging doctors to prescribe its drugs with free golf, massages, and junkets to posh resorts. [4]
- Eli Lilly & Co. agreed to pay $1.4 billion to settle similar charges involving its antipsychotic medicine Zyprexa. [5]
Decision
Global health care giant GlaxoSmithKline LLC (GSK) agreed to plead guilty and to pay $3 billion to resolve its criminal and civil liability arising from the company’s unlawful promotion of certain prescription drugs, its failure to report certain safety data, and its civil liability for alleged false price reporting practices, the Justice Department announced. The resolution is the largest health care fraud settlement in U.S. history and the largest payment ever by a drug company. [1]
- GSK agreed to plead guilty to three misdemeanor criminal counts, one each related to the three drugs. [3]
- Prosecutors have not brought criminal charges against any individuals in connection with the GSK case, although the settlement expressly leaves open that possibility. Cole declined to comment on the possibility of future charges. [3]
- Glaxo was one of the industry’s biggest marketing machines, with a thousands-strong sales force paid according to the number of prescriptions they could persuade doctors to write. As part of the deal, Glaxo agreed to change the way its sales representatives are compensated and remove sales goals for specific territories. Prosecutors said that compensation system was a driving force behind much of the misconduct in the case. [5]
- Prosecutors said they didn’t know how much the company made through its off-label promotions of its drugs, so it is possible the revenue generated by the practice exceeded the $3 billion penalty. [5]
Fines
Of the penalties, $1 billion covers criminal fines and forfeitures and $2 billion is for civil settlements with the federal government and the state governments of Massachusetts and Colorado. [4]
Integrity Agreement
As part of its deal, Glaxo has entered into a five-year “corporate integrity agreement” with the government in which it pledges to adhere to specific behavior. Corporate-integrity agreements are common in such settlements, but this one includes new features, including an “executive financial recoupment program,” which requires Glaxo to change its executive-compensation policies to permit the company to recoup annual bonuses and long-term incentives from certain executives if they, or their subordinates, engage in significant misconduct. Glaxo may claw back up to three years of annual performance pay from executives who are current employees and from those who have left the company. [5]
The company will not be able to compensate its salesmen based on sales goals for territories. It was also required to change its executive compensation program to allow the company to “claw back” certain pay for those engaged in misconduct. [3]
“Our five-year integrity agreement with GlaxoSmithKline requires individual accountability of its board and executives,” said Daniel R. Levinson, Inspector General of the U.S. Department of Health and Human Services. “For example, company executives may have to forfeit annual bonuses if they or their subordinates engage in significant misconduct, and sales agents are now being paid based on quality of service rather than sales targets.” [1]
Criminal Plea Agreement
Under the provisions of the Food, Drug and Cosmetic Act, a company in its application to the FDA must specify each intended use of a drug. After the FDA approves the product as safe and effective for a specified use, a company’s promotional activities must be limited to the intended uses that FDA approved. In fact, promotion by the manufacturer for other uses – known as “off-label uses” – renders the product “misbranded.” [1]
Paxil: In the criminal information, the government alleges that, from April 1998 to August 2003, GSK unlawfully promoted Paxil for treating depression in patients under age 18, even though the FDA has never approved it for pediatric use. The United States alleges that, among other things, GSK participated in preparing, publishing and distributing a misleading medical journal article that misreported that a clinical trial of Paxil demonstrated efficacy in the treatment of depression in patients under age 18, when the study failed to demonstrate efficacy. At the same time, the United States alleges, GSK did not make available data from two other studies in which Paxil also failed to demonstrate efficacy in treating depression in patients under 18. The United States further alleges that GSK sponsored dinner programs, lunch programs, spa programs and similar activities to promote the use of Paxil in children and adolescents. GSK paid a speaker to talk to an audience of doctors and paid for the meal or spa treatment for the doctors who attended. Since 2004, Paxil, like other antidepressants, included on its label a “black box warning” stating that antidepressants may increase the risk of suicidal thinking and behavior in short-term studies in patients under age 18. GSK agreed to plead guilty to misbranding Paxil in that its labeling was false and misleading regarding the use of Paxil for patients under 18. [1]
Wellbutrin: The United States also alleges that, from January 1999 to December 2003, GSK promoted Wellbutrin, approved at that time only for Major Depressive Disorder, for weight loss, the treatment of sexual dysfunction, substance addictions and Attention Deficit Hyperactivity Disorder, among other off-label uses. The United States contends that GSK paid millions of dollars to doctors to speak at and attend meetings, sometimes at lavish resorts, at which the off-label uses of Wellbutrin were routinely promoted and also used sales representatives, sham advisory boards, and supposedly independent Continuing Medical Education (CME) programs to promote Wllbutrin for these unapproved uses. GSK has agreed to plead guilty to misbranding Wellbutrin in that its labeling did not bear adequate directions for these off-label uses. For the Paxil and Wellbutrin misbranding offenses, GSK has agreed to pay a criminal fine and forfeiture of $757,387,200. [1]
Avandia: The United States alleges that, between 2001 and 2007, GSK failed to include certain safety data about Avandia, a diabetes drug, in reports to the FDA that are meant to allow the FDA to determine if a drug continues to be safe for its approved indications and to spot drug safety trends. The missing information included data regarding certain post-marketing studies, as well as data regarding two studies undertaken in response to European regulators’ concerns about the cardiovascular safety of Avandia. Since 2007, the FDA has added two black box warnings to the Avandia label to alert physicians about the potential increased risk of (1) congestive heart failure, and (2) myocardial infarction (heart attack). GSK has agreed to plead guilty to failing to report data to the FDA and has agreed to pay a criminal fine in the amount of $242,612,800 for its unlawful conduct concerning Avandia. [1]
Civil Settlement Agreement
The drug corporation also agreed to resolve civil liability for promoting the drugs Paxil, Wellbutrin, Advair, Lamictal and Zofran for off-label, non-covered uses. In the civil settlement, the government said Advair was promoted as a first-line therapy for mild asthma though not approved for that and for chronic obstructive pulmonary disease with misleading claims. It said the anti-epileptic medicine Lamictal was promoted for off-label psychiatric uses, neuropathic pain and pain management. And it said certain forms of Zofran, approved only for post-operative nausea, were promoted to treatment of morning sickness in pregnant women. [4]
The company also resolved accusations that it paid kickbacks to doctors to prescribe those drugs as well as the drugs Imitrex, Lotronex, Flovent and Valtrex. [4]
The civil portion of the settlement relates to conduct the government alleges led taxpayer-funded health programs, including Medicaid, to overpay for Glaxo drugs. [5]
Part of civil fines address allegations that, from 1994 to 2003, GSK underpaid money owed to Medicaid, the healthcare program for the poor run jointly by states and the federal government. The company had an obligation to tell the government its “best prices” but failed to do so, prosecutors said, and $300 million of the settlement will go to states and other public health authorities. [3]
A portion of the $2 billion in civil fines may go to a group of whistleblowers who contributed to the government’s investigation and who are eligible to share in the recovery under the False Claims Act. Cole said the amount has not been determined. [3]
As part of this global resolution, GSK has agreed to resolve its civil liability for the following alleged conduct: (1) promoting the drugs Paxil, Wellbutrin, Advair, Lamictal and Zofran for off-label, non-covered uses and paying kickbacks to physicians to prescribe those drugs as well as the drugs Imitrex, Lotronex, Flovent and Valtrex; (2) making false and misleading statements concerning the safety of Avandia; and (3) reporting false best prices and underpaying rebates owed under the Medicaid Drug Rebate Program. [1]
Off-Label Promotion and Kickbacks: The civil settlement resolves claims set forth in a complaint filed by the United States alleging that, in addition to promoting the drugs Paxil and Wellbutrin for unapproved, non-covered uses, GSK also promoted its asthma drug, Advair, for first-line therapy for mild asthma patients even though it was not approvedor medically appropriate under these circumstances. GSK also promoted Advair for chronic obstructive pulmonary disease with misleading claims as to the relevant treatment guidelines. The civil settlement also resolves allegations that GSK promoted Lamictal, an anti-epileptic medication, for off-label, non-covered psychiatric uses, neuropathic pain and pain management. It further resolves allegations that GSK promoted certain forms of Zofran, approved only for post-operative nausea, for the treatment of morning sickness in pregnant women. It also includes allegations that GSK paid kickbacks to health care professionals to induce them to promote and prescribe these drugs as well as the drugs Imitrex, Lotronex, Flovent and Valtrex. The United States alleges that this conduct caused false claims to be submitted to federal health care programs. [1]
This off-label civil settlement resolves four lawsuits pending in federal court in the District of Massachusetts under the qui tam, or whistleblower, provisions of the False Claims Act, which allow private citizens to bring civil actions on behalf of the United States and share in any recovery. [1]
Avandia: In its civil settlement agreement, the United States alleges that GSK promoted Avandia to physicians and other health care providers with false and misleading representations about Avandia’s safety profile, causing false claims to be submitted to federal health care programs. Specifically, the United States alleges that GSK stated that Avandia had a positive cholesterol profile despite having no well-controlled studies to support that message. The United States also alleges that the company sponsored programs suggesting cardiovascular benefits from Avandia therapy despite warnings on the FDA-approved label regarding cardiovascular risks. GSK has agreed to pay $657 million relating to false claims arising from misrepresentations about Avandia. The federal share of this settlement is $508 million and the state share is $149 million. [1]
Price Reporting: GSK is also resolving allegations that, between 1994 and 2003, GSK and its corporate predecessors reported false drug prices, which resulted in GSK’s underpaying rebates owed under theMedicaid Drug Rebate Program. By law, GSK was required to report the lowest, or “best” price that it charged its customers and to pay quarterly rebates to the states based on those reported prices. When drugs are sold to purchasers in contingent arrangements known as “bundles,” the discounts offered for the bundled drugs must be reallocated across all products in the bundle proportionate to the dollar value of the units sold. The United States alleges that GSK had bundled sales arrangements that included steep discounts known as “nominal” pricing and yet failed to take such contingent arrangements into account when calculating and reporting its best prices to the Department of Health and Human Services. Had it done so, the effective prices on certain drugs would have been different, and, in some instances, triggered a new, lower best price than what GSK reported. As a result, GSK underpaid rebates due to Medicaid and overcharged certain Public Health Service entities for its drugs, the United States contends. GSK has agreed to pay $300 million to resolve these allegations, including $160,972,069 to the federal government, $118,792,931 to thestates, and $20,235,000 to certain Public Health Service entities who paid inflated prices for the drugs at issue. [1]
Aftermath
Plaintiffs/ Government Reaction
Deputy Attorney General James M. Cole
Let me be clear: we will not tolerate health care fraud. And, in every instance where we uncover it, we will use all available tools to hold those responsible to account. [2] said Deputy Attorney General James M. Cole Speaks at the GSK Press Conference
The $3 billion combined criminal-civil fine will be the largest penalty ever paid by a drug company, Deputy Attorney General James M. Cole said. The corporation also agreed to be monitored by government officials for five years to attempt to ensure the company’s compliance, Cole said. [4]
“Today’s multi-billion dollar settlement is unprecedented in both size and scope. It underscores the Administration’s firm commitment to protecting the American people and holding accountable those who commit health care fraud,” said James M. Cole, Deputy Attorney General. “At every level, we are determined to stop practices that jeopardize patients’ health, harm taxpayers, and violate the public trust – and this historic action is a clear warning to any company that chooses to break the law.” [1]
“Today’s historic settlement is a major milestone in our efforts to stamp out health care fraud,” said Bill Corr, Deputy Secretary of the Department of Health and Human Services (HHS). “For a long time, our health care system had been a target for cheaters who thought they could make an easy profit at the expense of public safety, taxpayers, and the millions of Americans who depend on programs like Medicare and Medicaid. But thanks to strong enforcement actions like those we have announced today, that equation is rapidly changing.” [1]
This resolution marks the culmination of an extensive investigation by special agents from HHS-OIG, FDA and FBI, along with law enforcement partners across the federal government. Moving forward, GSK will be subject to stringent requirements under its corporate integrity agreement with HHS-OIG; this agreement is designed to increase accountability and transparency and prevent future fraud and abuse. Effective law enforcement partnerships and fraud prevention are hallmarks of the Health Care Fraud Prevention and Enforcement Action Team (HEAT) initiative, which fosters government collaboration to fight fraud. [1]
Acting Assistant Attorney General Stuart F. Delery,
“For far too long, we have heard that the pharmaceutical industry views these settlements merely as the cost of doing business,” Acting Assistant Attorney General Stuart F. Delery, head of Justice’s civil division, said at the news conference. “That is why this administration is committed to using every available tool to defeat health care fraud.” [4]
Delery added, “Today’s resolution seeks not only to punish wrongdoing and recover taxpayer dollars, but to ensure GSK’s future compliance with the law.” He noted that a similar recent settlement with Abbott Laboratories also included continuing compliance monitoring. [4]
“This landmark settlement demonstrates the Department’s commitment to protecting the American public against illegal conduct and fraud by pharmaceutical companies,” said Stuart F. Delery, Acting Assistant Attorney General for the Justice Department’s Civil Division. “Doctors need truthful, fair, balanced information when deciding whether the benefits of a drug outweigh its safety risks. By the same token, the FDA needs all necessary safety-related information to identify safety trends and to determine whether a drug is safe and effective. Unlawful promotion of drugs for unapproved uses and failing to report adverse drug experiences to the FDA can tip the balance of those important decisions, and the Justice Department will not tolerate attempts by those who seek to corrupt our health care system in this way.” [1]
Deborah M. Autor, Esq., Deputy Commissioner for Global Regulatory Operations and Policy,US FDA
“The FDA Office of Criminal Investigations will aggressively pursue pharmaceutical companies that choose to put profits before the public’s health,” said Deborah M. Autor, Esq., Deputy Commissioner for Global Regulatory Operations and Policy, U.S. Food and Drug Administration. “We will continue to work with the Justice Department and our law enforcement counterparts to target companies that disregard the protections of the drug approval process by promoting drugs for uses when they have not been proven to be safe and effective for those uses, and that fail to report required drug safety information to the FDA.” [1]
Carmen Ortiz, U.S. Attorney for the District of Massachusetts
“This case demonstrates our continuing commitment to ensuring that the messages provided by drug manufacturers to physicians and patients are true and accurate and that decisions as to what drugs are prescribed to sick patients are based on best medical judgments, not false and misleading claims or improper financial inducements,” said Carmen Ortiz, U.S. Attorney for the District of Massachusetts. [1]
John Walsh, U.S. Attorney for Colorado.
“Patients rely on their physicians to prescribe the drugs they need,” said John Walsh, U.S. Attorney for Colorado. “The pharmaceutical industries’ drive for profits can distort the information provided to physicians concerning drugs. This case will help to ensure that your physician will make prescribing decisions based on good science and not on misinformation, money or favors provided by the pharmaceutical industry.” [1]
Defendant/ GlaxoSmithKline Reaction
GlaxoSmithKline CEO Sir Andrew Witty expressed regret and said they have learned “from the mistakes that were made.” [4]
“Today brings to resolution difficult, long-standing matters for GSK,” he said in a statement. “Whilst these originate in a different era for the company, they cannot and will not be ignored.” [4]
In a statement, GlaxoSmithKline said it disagreed with some statements the Justice Department made in court papers. For example, the company said its settlement with the government does “not constitute an admission of any liability or wrongdoing in the selling and marketing of Lamictal, Zofran, Imitrex, Lotronex, Flovent, Valtrex, Avandia or Advair products. The government also made allegations about Paxil, Wellbutrin that the company did not admit.” [4]
“The civil settlement agreement contains many allegations that are either inaccurate or incomplete, that selectively tell only parts of the story, and that draw unwarranted conclusions from disputed facts,” the company said. [4]
Further Research
Court Documents:
- US Complaint
- GSK Criminal Information
- HHS-OIG Corporate Integrity Agreement
- Civil Avandia Settlement Agreement
- Civil Nominals Settlement Agreement
- Civil Off Label Settlement Agreement
In the news:
Media
Glaxo SmithKline $3 billion fraud settlement- 2012
source: PharmacognosiaTV
GlaxoSmithKline (GSK) Fined $3 Billion 2012
source: The Young Turks
$3B GlaxoSmithKline Whistleblower Settlement – 2012
source: Sheller, PC
GSK in China – where now? – 2009
source: Financial Times
References
- GlaxoSmithKline to Plead Guilty and Pay $3 Billion to Resolve Fraud Allegations and Failure to Report Safety Data
- Deputy Attorney General James M. Cole Speaks at the GSK Press Conference
- GlaxoSmithKline settles healthcare fraud case for $3 billion
- GlaxoSmithKline to plead guilty, pay $3B for illicit promotion of drugs in record settlement
- Glaxo in $3 Billion Settlement
- Drugwatch: GlaxoSmithKline (GSK)
Keyword
???
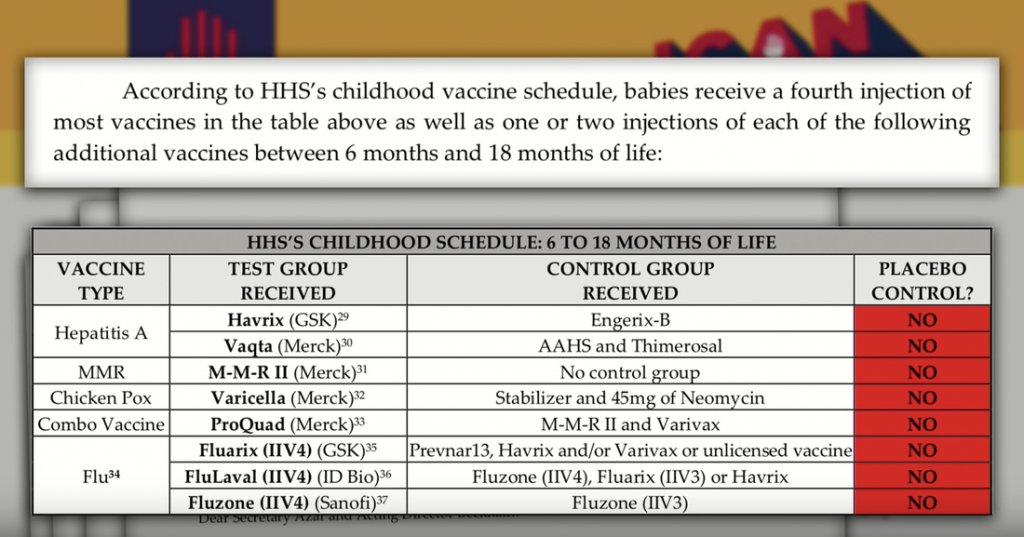
![[SOURCE] Page 14 ICAN Response December 31, 2018](http://coronacases.wiki/wp-content/uploads/2022/04/screen-shot-2019-01-27-at-3-44-49-pm_orig-1024x352.png)