Vaccine Safety Records Case
Vaccine Safety Records Case
Re: the Legality of not providing Covid Vaccine Safety Records in response to a FOIA
Facts of the Case
- Dates: Nov 1, 2022 (filed)
- Location: Washington DC, USA
- Court: US District Court for DC
- Case #: 1:22-cv-03153
- Plaintiff: Judicial Watch
- Plaintiff’s Lawyer: Tom Fitton
- Defendant: U.S. Dept of Health & Human Services (HHS)
- Trial Type: FOIA
- Judge:
- Status: Ongoing
- Verdict: TBD
*updated Nov 11, 2022
Background
Judicial Watch filed a Freedom of Information Act (FOIA) lawsuit against the U.S. Department of Health and Human Services (HHS) for records on COVID-19 vaccine safety studies (Judicial Watch, Inc. v. U.S. Department of Health and Human Services ) [1]
Plaintiff Judicial Watch, Inc. is a not-for-profit, educational organization that seeks to promote transparency, integrity, and
accountability in government and fidelity to the rule of law. As part of its mission, Plaintiff regularly requests records from federal agencies, analyzes the responses it receives, and disseminates its findings and the records to the American public to inform them about ”what their government is up to. [1]
The lawsuit was filed in the U.S. District Court for the District of Columbia after National Institutes of Allergies and Infectious Diseases (a component of HHS) inadequately responded to a June 1, 2022, Judicial Watch FOIA request for: [1]
- All safety studies, data, reports, and analyses produced by the Division of Microbiology and Infectious Diseases (DMID) relating to the safety of ‘vaccines’ and/or gene therapies to treat and/or prevent SARS-CoV-2 and/or COVID-19 made by Pfizer, BioNTech, Moderna, Johnson & Johnson, and Janssen. [1]
- All emails sent to and from the following DMID officials relating to the safety of ‘vaccines’ and/or gene therapies to treat and/or prevent SARSCoV-2 and/or COVID-19 made by Pfizer, BioNTech, Moderna, Johnson & Johnson, and Janssen: [1]
- The Director of DMID
- The head of the Office of Genomics & Advanced Technologies
- The head of the Office of International Research in Infectious Diseases
- The head of the Office of Regulatory Affairs
- The head of the Office of Clinical Research Affairs
- The head of the Clinical Trials Management Section
- The head of the Virology Branch
- The head of the Respiratory Diseases Branch
- The head of the Influenza, SARS, and Other Viral Respiratory Diseases Section
On May 3, 2022, the National Institutes of Health (NIH) released a paper titled “Safety and Immunogenicity of a Third Dose of SARS-CoV-2 mRNA Vaccine – An Interim Analysis” that “evaluated early safety and immunogenicity after a third mRNA vaccination in adults who received the mRNA-1273 primary series in the Phase 1 trial approximately 9 to 10 months earlier.” Contributors to that study include three affiliates of the DMID: Mamodikoe Makhene (DMID medical officer), Wendy Buchanan (DMID Clinical Project Manager) and Paul Roberts (DMID Chief Respiratory Pathogens Clinical Research). [1]
“The Biden administration is playing shell games with documents on the Covid vaccine,” stated Judicial Watch President Tom Fitton. “The arrogant cover-up of COVID vaccine safety information further undermines public confidence in these already controversial drugs.” [1]
on June 1, 2022 NIAID acknowledged receipt of the FDA request and assigned
the request No. 58421. [2]
Between June 21, 2022 and June 27, 2022, Plaintiff and a policy analyst from Defendant’s FOIA department agreed to narrow the scope of the request. Defendant never responded to Plaintiff’s June 27,2022 email. [2]
As of the date of this Complaint, Defendant has failed to: [2]
(i) determine whether to comply with the request;
(ii) notify Plaintiff of any such determination or the reasons therefor;
(iii) advise Plaintiff of the right to appeal any adverse determination; or
(iv) produce the requested records or otherwise demonstrate that the requested records are exempt from
production.
WHEREFORE, Plaintiff respectfully requests that the Court: [2]
(1) order Defendant to conduct a search for any and all records responsive to Plaintiff’s FOIA requests and demonstrate that it employed search methods reasonably likely to lead to the discovery of records responsive to Plaintiff’s FOIA requests;
(2) order Defendant to produce, by a date certain, any and all non-exempt records responsive to Plaintiff’s FOIA requests and Vaughn indices of any responsive records withheld under claim of exemption;
(3) enjoin Defendant from continuing to withhold any and all non-exempt records responsive to Plaintiff’s FOIA requests;
(4) grant Plaintiff an award of attorneys’ fees and other litigation costs reasonably incurred in this action pursuant to 5 U.S.C. § 552(a)(4)(E); and (5) grant Plaintiff such other relief as the Court deems just and proper.
Significance
The release of the Covid Vaccine Safety data may – like other granted FOIA requests – may show that the vaccines are not as advertised and may halt the distribution of these injections to an unknowing public. Furthermore if there was criminal fraud there may be convictions.
Plaintiff’s Argument
…More information is needed…
Defendant’s Argument
…More information is needed…
Relevant Prior Judgements/ Cases
Prior FOIA requests have led to data that clearly show that the covid injection trials were fraught with irregularities and improper methods that are likely fraudulent. This is likely why Pfizer attempted to block the release of these documents for 75 years. Thankfully a judge ordered Pfizer to release the docs.
Other FOIAs have shown similar evidence
Through previous FOIA activity, Judicial Watch has uncovered a substantial amount of information about COVID-19 issues: [1]
- In October, Judicial Watch uncovered FDA records regarding the COVID booster vaccines through a FOIA lawsuit for records of communication from the former director and deputy director of the FDA’s Office of Vaccines Research and Review, Dr. Marion Gruber and Dr. Philip Krause. On September 13, 2021, Gruber and Krause were among a group of resigning doctors who agreed that,
“Available evidence doesn’t yet indicate a need for COVID-19 vaccine booster shots among the general population …”
- In July 2022, NIH records revealed an FBI “inquiry” into the NIH’s controversial bat coronavirus grant tied to the Wuhan Institute of Virology. The records also show National Institute of Allergy and Infectious Diseases (NIAID) officials were concerned about “gain-of-function” research in China’s Wuhan Institute of Virology in 2016. The Fauci agency was also concerned about EcoHealth Alliance’s lack of compliance with reporting rules and use of gain-of-function research in the NIH-funded research involving bat coronaviruses in Wuhan, China.
- FDA records showed top officials being pressured by companies and the Biden administration to impose timelines on approval for the booster shots “that make no sense”
- HHS records revealed that from 2014 to 2019, $826,277 was given to the Wuhan Institute of Virology for bat coronavirus research by the NIAID.
- NIAID records showed that it gave nine China-related grants to EcoHealth Alliance to research coronavirus emergence in bats and was the NIH’s top issuer of grants to the Wuhan lab itself. The records also included an email from the vice director of the Wuhan Lab asking an NIH official for help finding disinfectants for decontamination of airtight suits and indoor surfaces.
- HHS records included an “urgent for Dr. Fauci ” email chain, citing ties between the Wuhan lab and the taxpayer-funded EcoHealth Alliance. The government emails also reported that the foundation of U.S. billionaire Bill Gates worked closely with the Chinese government to pave the way for Chinese-produced medications to be sold outside China and help “raise China’s voice of governance by placing representatives from China on important international counsels as high level commitment from China.”
- HHS records included a grant application for research involving the coronavirus that appears to describe “gain-of-function” research involving RNA extractions from bats, experiments on viruses, attempts to develop a chimeric virus and efforts to genetically manipulate the full-length bat SARSr-CoV WIV1 strain molecular clone.
- HHS records showed the State Department and NIAID knew immediately in January 2020 that China was withholding COVID data, which was hindering risk assessment and response by public health officials.
- University of Texas Medical Branch (UTMB) records show the former director of the Galveston National Laboratory at the University of Texas Medical Branch (UTMB), Dr. James W. Le Duc warned Chinese researchers at the Wuhan Institute of Virology of potential investigations into the COVID issue by Congress.
- HHS records regarding biodistribution studies and related data for the COVID-19 vaccines show a key component of the vaccines developed by Pfizer/BioNTech, lipid nanoparticles (LNPs), were found outside the injection site, mainly the liver, adrenal glands, spleen and ovaries of test animals, eight to 48 hours after injection.
- Records from the Federal Select Agent Program (FSAP) reveal safety lapses and violations at U.S. biosafety laboratories that conduct research on dangerous agents and toxins.
- HHS records include emails between National Institutes of Health (NIH) then-Director Francis Collins and Anthony Fauci, the director of National Institute of Allergy and Infectious Diseases (NIAID), about hydroxychloroquine and COVID-19.
- HHS records show that NIH officials tailored confidentiality forms to China’s terms and that the World Health Organization (WHO) conducted an unreleased, “strictly confidential” COVID-19 epidemiological analysis in January 2020.
- Fauci emails include his approval of a press release supportive of China’s response to the 2019 novel coronavirus.
Decision
…More information is needed…
Aftermath
…More information is needed…
Further Research
Court Documents:
In the news:
…More information is needed…
Media
References
Keyword
Biden, Division of Microbiology and Infectious Diseases, DMID, Fauci, Fitton, FOIA, Freedom of Information Act, Freedom of Information Act request, National Institute of Allergy and Infectious Diseases, National Institutes of Health, NIAID, NIH, Records, Safety, USA. Judicial Watch, Vaccine, Wuhan Institute of Virology
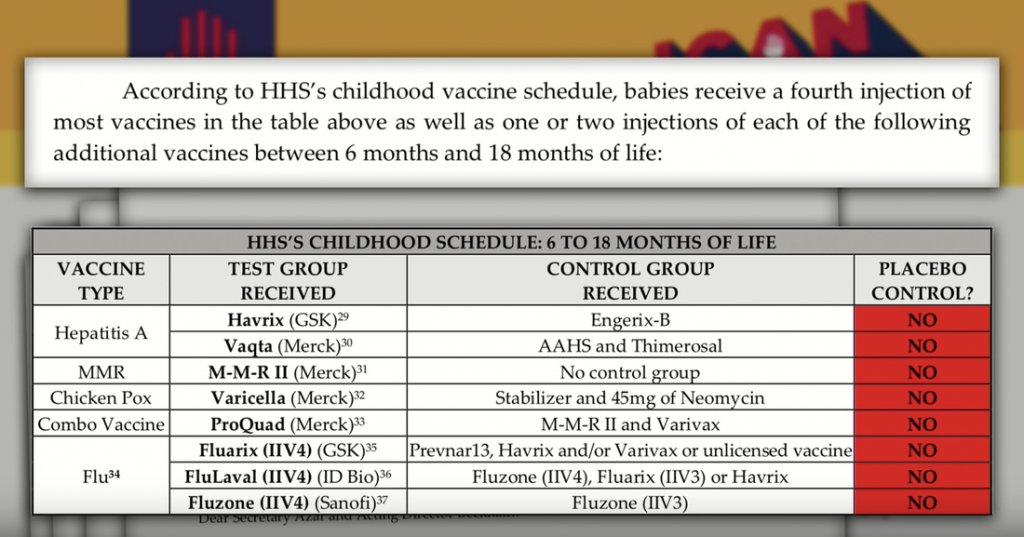
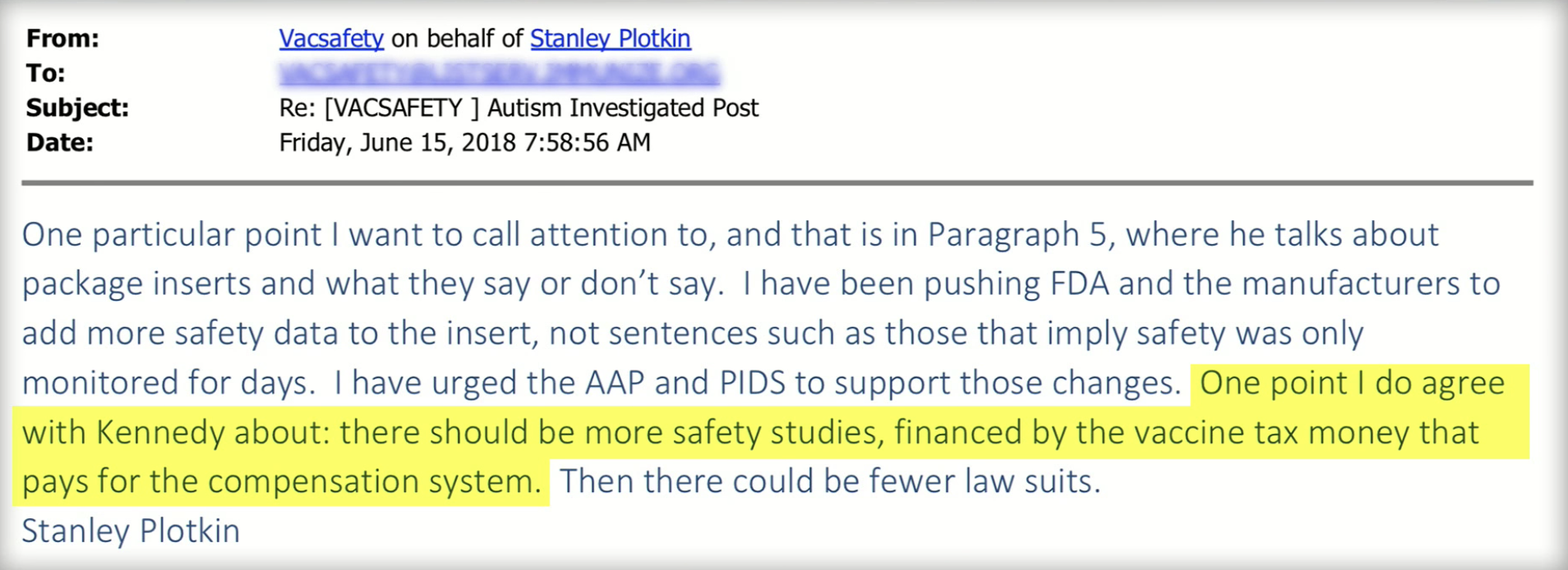
![[SOURCE] Page 14 ICAN Response December 31, 2018](http://coronacases.wiki/wp-content/uploads/2022/04/screen-shot-2019-01-27-at-3-44-49-pm_orig-1024x352.png)