ICAN Placebo FOIA
ICAN Placebo FOIA
Re: the data & proof of placebo control studies during vaccine clinical trials
Facts of the Case
- Dates: (filed) Oct 12, 2017
- Location: New York, USA
- Court: U.S. District Court for the Southern District of New York
- Case #:
- Plaintiff: ICAN
- Plaintiff’s Lawyer: Robert F. Kennedy, Jr.
- Defendant: HHS, FDA
- Trial Type: FOIA
- Judge:
- Status: Decided
- Verdict: for the Plaintiff
*updated: April 16, 2022
Background
The Department of Health and Human Services (HHS) was sent a 19-page legal notice on October 12, 2017 by the Informed Consent Action Network (ICAN) requesting confirmation that certain obligations regarding vaccine safety required under the 1986 Act had been fulfilled or will be fulfilled. [1]
Specifically at issue is the use of placebo’s (inert substances) during clinical safety trials
The notice asked (HHS) to “[p]lease explain how HHS justifies licensing any pediatric vaccine without first conducting a long-term clinical trial in which the rate of adverse reactions is compared between the subject group and a control group receiving an inert placebo?” [3]
ICAN sought copies of the reports HHS was required to submit to Congress every two years, starting in 1988, detailing improvements it made to vaccine safety. ICAN was represented by Robert F. Kennedy, Jr. [2]
ICAN was founded by Del Bigtree who hosts the weekly live online news program “The HighWire,” and is an Emmy-award winning producer of the CBS medical talk show “The Doctors.” He is producer of the groundbreaking documentary “Vaxxed: From Coverup to Catastrophe.” [2]
“The 1986 National Childhood Vaccination Injury Act granted economic immunity to pharmaceutical companies for vaccine injuries and hence eviscerated their economic incentive for them to take responsibility for vaccine safety,” says Bigtree. “Market forces driving vaccine safety were simply eliminated.” [2]
Congress therefore charged the Secretary of HHS with the explicit responsibility to assure vaccine safety. Biannual reports of HHS’s progress in improving vaccine safety were to be submitted to Congress. Yet, as ICAN has now proven, these reports were never created. [2]
The request included over 55 organizations whose members exceed five million Americans also voicing concern about vaccine safety. Freedom of Information Act (FOIA) documents show that the agency took the notice very seriously calling upon all internal departments and resources to craft their response. According to the FOIA documents, all HHS operating divisions, The National Vaccine Program Office, Assistant Secretary for Financial Resources, and The Office of the Assistant Secretary of Health all took part in the response. In Addition, FOIA email documents also state, “The National Vaccine Program Office staff has pulled together a draft response and is requesting that it be cleared with CDC, OGC, FDA, HRSA, NIH, and AHRQ for review prior to signature.” [1]
Despite nine different government entities working on the response, their eventual January 18, 2018 reply was both lackluster and telling. Their brief 9-page response was unable to provide solid answers to the eleven questions asked of the agency concerning vaccine safety. The flimsily HHS reply triggered a deeply thorough 88-page response from ICAN laying out every detail concerning the problems and issues happening in regards to vaccine safety stemming from HHS. Its opening page writes: [1]
“Given the gravity of HHS’s responsibility, it is deeply troubling that the majority of HHS’s letter contains little more than broad unsupported conclusory assertions. Most of these conclusory assertions do not withstand basic scrutiny. HHS’s responses even often contradict its own source materials.” [1]
Among several concerning points, HHS choose to began their January reply by writing, “…many pediatric vaccines have been investigated in clinical trials that included a placebo.” [1]
As defined by the CDC, a “placebo” is: “A substance or treatment that has no effect on human beings.” [1]
HHS’s response also claimed that safety in these trials was reviewed for a significant duration, without specifying any duration. [3]
Recently on his show The HighWire, host Del Bigtree began publicly unpacking ICAN’s 88-page response by focusing on pre-licensure vaccine safety trials. The HHS reply contradicted itself by first admitting,
“Inert placebo controls are not required to understand the safety profile of a new vaccine, and are thus not required. In some cases, inclusion of placebo control groups is considered unethical.”
Later in their same letter, HHS states, “Vaccines are held to strict standards of safety.” [1]
Bigtree stated: “They’ve now printed it. So now you know for a fact that they just admitted those products you’re injecting into your kids have never been compared to a control. An inert saline injection.” [1]
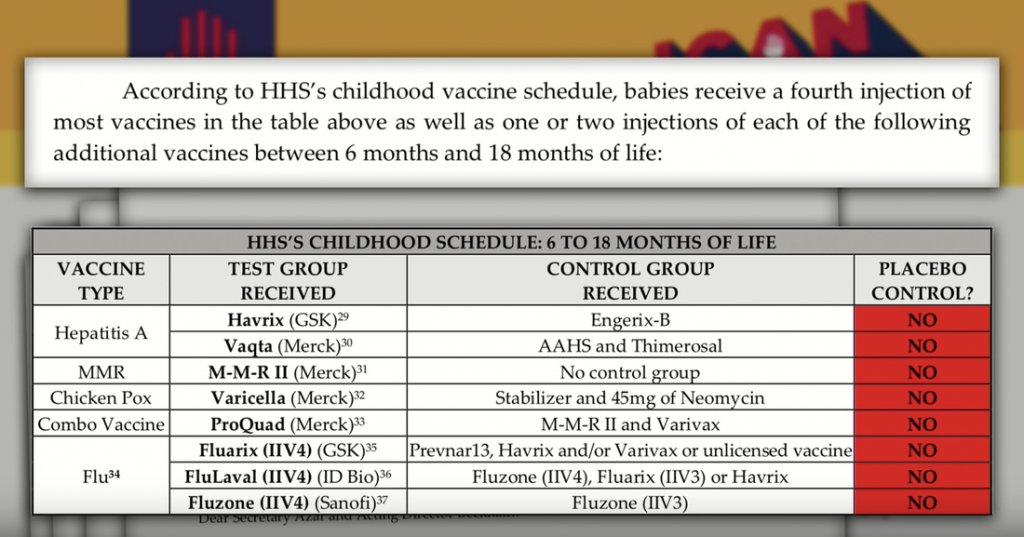
Furthermore, according to HHS’s childhood vaccine schedule, babies receive 3 injections of each of the following vaccines between day one and 6 months of life all untested against a placebo control. [1]
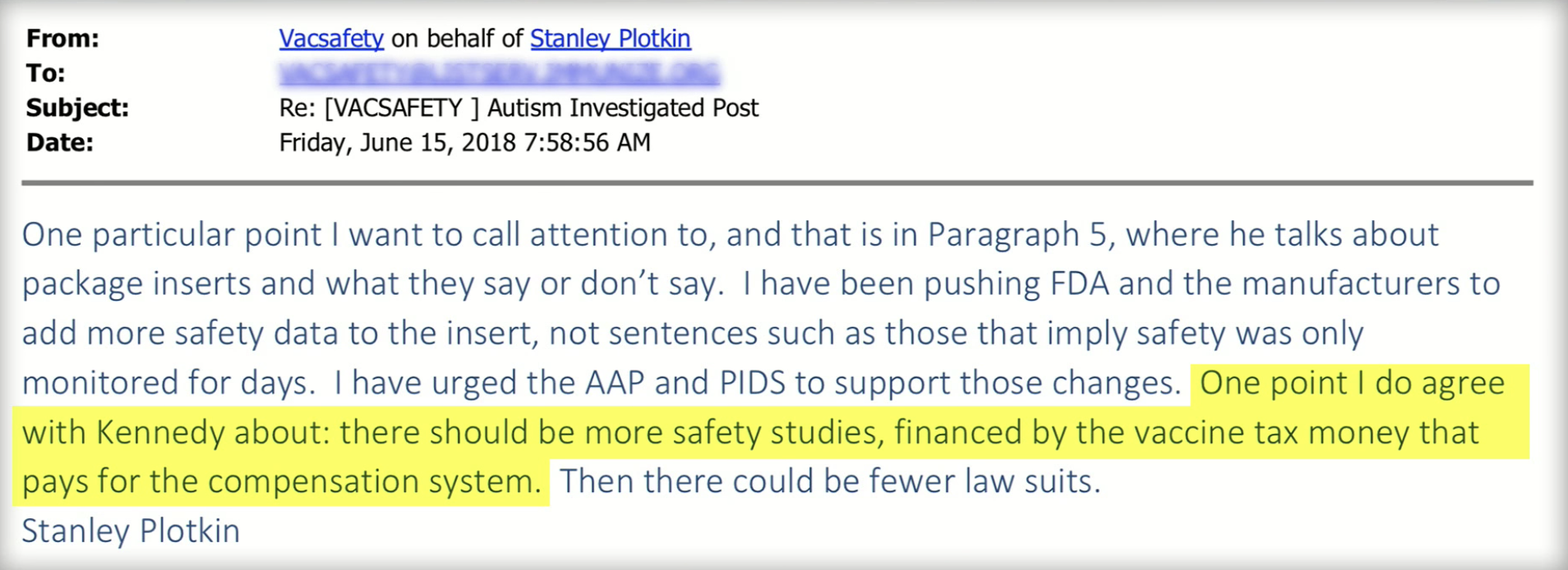
Even pro-vaccine luminaries like Dr. Stanley Plotkin, vaccine authority and author of Plotkin’s Vaccines, appears to be in agreement with ICAN. Emails from a recent FOIA request show Plotkin wrote in June 2018 correspondence, “One point I do agree with [Robert F] Kennedy [Jr] about: there should be more safety studies…” [1]
Significance
Vaccine manufacturers insist that their products are safe and effective, despite many in the public reporting otherwise (injuries). Control experiments are essential to understanding how a medical product performs. Until this FOIA request, there has not been a public disclosure of this information casting doubt as to the “Science” underpinning the safe and effective claim. This information could harm the faith in one or all vaccines, and could be potentially destructive to this industry.
Plaintiff’s Argument
Given the conflicting information coming from HHS, ICAN’s 88-page response pressured their assertions writing: [1]
“It is troubling that HHS chose to begin its response by misstating that prior to licensure for children “ many pediatric vaccines have been investigated in clinical trials that included a placebo.” At worst, HHS knowingly perpetuated this inaccurate claim but at best, HHS was unaware this claim was incorrect. This leaves the public to wonder what other critical assumptions underpinning HHS’s confidence in vaccine safety are incorrect.” [1]
ICAN’s original HHS notice directly pointed to concerns about the two Hepatitis B vaccines licensed for injection into one-day-old babies. Namely that the vaccines, according to the FDA’s own vaccine insert included in the original HHS notice, show that not only were the shots not tested against inert placebos, both vaccines were licensed after trials that solicited adverse reactions for only four days [Merck] and five days [GlaxoSmithKline] respectively after vaccination. To this point, HHS responded by referring back to the same inserts which contained the concerning data by simply writing, “Data relied upon in licensing infant use of hepatitis B vaccines is summarized in the respective package inserts.” [1]
ICAN’s response included a detailed chart containing every pediatric vaccine, citing to FDA documents, which indisputably proves that it was categorically false for HHS and the FDA to claim that “many pediatric vaccines have been investigated in clinical trials that included a placebo.” [3]
Perhaps one of the most damning findings referred to in ICAN’s 88-page HHS letter was titled HHS’s “Safety” Pyramid Scheme. Since inert placebo controls are not required and rarely used in vaccine safety testing, other vaccines take that role in what are referred to as “active controls.” ICAN’s 88-page response to HHS points out to the agency the following: [1]
“HHS’s own industry guidance for drug testing explains that an active control is only appropriate if it is a “drug whose effect is well-defined,” which means “historical placebo-controlled trials are available to define the active control effect.” Despite its own policy and guidance, HHS does not require this minimal assurance for vaccines.” [1]
![[SOURCE] Page 14 ICAN Response December 31, 2018](http://coronacases.wiki/wp-content/uploads/2022/04/screen-shot-2019-01-27-at-3-44-49-pm_orig-1024x352.png)
Defendant’s Argument
…More information is needed…
Decision
The US Department of Health and Human Services (HHS) settled a court-ordered stipulation admitting that they could not produce 30 years of required evidence showing they have made improvements in the manufacturing, testing, warning, field surveillance, adverse reaction reporting and research on vaccines in order to reduce the risk of adverse reactions as mandated by the 1986 National Childhood Vaccine Injury Act (1986 Act). [1]
LOS ANGELES, Sept. 14, 2018 /PRNewswire/ — The U.S. Department of Health and Human Services (HHS) has admitted that, in direct violation of Federal law, it failed to provide a single vaccine safety report to Congress for thirty years, according to Informed Consent Action Network (ICAN).
HHS conceded that those reports do not exist and the Court entered an order confirming this concession. [2]
Aftermath
ICAN’s response:
“It is apparent that HHS doesn’t have a clue as to the actual safety profile of the now 39 doses, and growing, of vaccines given by one year of age, including in utero,” said Bigtree. “In 1986, a one-year old child received eleven doses. [2]
“HHS spends billions annually promoting vaccines and generates a steady stream of reports promoting vaccines,” Bigtree says. “Yet, when, despite Federal law, HHS cannot bother to complete the simple task of preparing a biennial report on vaccine safety, there is little hope HHS is tackling the much harder job of improving vaccine safety.” [2]
The 1986 Act shifted financial liability for vaccine injuries to the U.S. Government which has since 1986 paid over $3.9 billion for serious vaccine injuries. [2]
The reality is that none – save one – of the pediatric vaccines was licensed based on a placebo controlled clinical trial! … No proof has ever been provided. [3]
Further ICAN Lawsuits
- ICAN also sued the FDA numerous times to force it to release clinical trial reports for other vaccines, including, for example, the chicken pox vaccine, which we explained in another recent legal update.
- when the Phase III trial for AstraZeneca and the University of Oxford’s COVID-19 vaccine was underway in England using another vaccine (Menveo) as a control (instead of a placebo), ICAN filed a forceful petition demanding that the FDA mandate that all clinical trials of COVID-19 vaccines use a placebo control as well as track safety long-term in a properly sized trial.
Nine days after ICAN filed its petition, on June 30, 2020, the FDA changed course and issued emergency guidance to industry that all COVID-19 clinical trials must use a placebo control.
- On July 17, 2020, ICAN sued the FDA in federal court demanding the entire clinical trial report for Menveo, just in case the agency was considering permitting this vaccine as a control in the AstraZeneca trial to be conducted in the United States. On July 20, 2020, ICAN also filed a forceful amended petition with the FDA thanking it for requiring a placebo control group but demanding, among other things, that it also require that these clinical trials track all adverse events during the entire duration of the trial – not just for a limited time period.
- Not long thereafter, in mid-September, in a highly unusual move, the full clinical trial protocols for the COVID-19 vaccines for which ICAN filed its petitions were released to the public. See copies for each of the manufacturer’s vaccines: AstraZeneca, Pfizer, Moderna, and Johnson & Johnson. Those protocols revealed that some of ICAN’s demands regarding the duration for tracking vaccine safety had been met.
Further Research
Court Documents:
- Read the Court Ruling
- ICAN 19-page legal notice to HHS (Oct 12, 2017)
- HHS January 18, 2018 reply
- 88-page response from ICAN
- 1986 National Childhood Vaccine Injury Act
More from ICAN
- ICAN Official Site: Legal Actions
- CDC Cannot Provide an Instance of a Single Confirmed COVID-19 Death in a Child Younger Than 16
- ICAN-Obtained Study Shows Adverse Events Increase When Chickenpox and MMR Vaccines are Given at the Same Time
- Pfizer’s Own Informed Consent Documents Undermine FDA and CDC’s Cries of “Safe and Effective”
Media
References
- The US Government Loses the Vaccine Debate
- ICAN vs. HHS: Key Legal Win Recasts Vaccine Debate
- ICAN v. FDA – ICAN Brings Lawsuit Related to “Placebo” in COVID-19 Clinical Trial
Keyword
1986, Adverse Reaction, Babies, Bigtree, Congress, Control, Del, Experiment, FDA, Field Surveillance, FOIA, Freedom of Information Act, GlaxoSmithKline, Health and Human Services, Hepatitis B, HHS, HighWire, ICAN, Informed Consent Action Network, Injection, Kennedy, Manufacturing, Merck, National Childhood Vaccine Injury Act, Nonprofit, Placebo, Plotkin, Reporting, RFK, Risk, Testing, US Department of, Warning