Vaccine Mandate Case: Supreme Court
Vaccine Mandate Case: Supreme Court
Re: the Legality of Vaccine Mandates
Facts of the Case
- Dates: May 2, 2022 (decided)
- Location: India
- Court: Supreme Court
- Case #:
- Petitioner: Dr.Jacaob Puliyel
- Petitioner’s Lawyer: Prashant Bhushan
- Respondent: Union & State Governments of Delhi, Madhya Pradesh, Maharashtra & Tamil Nadu
- Trial Type: Supreme Court
- Justices: L Nageswara Rao & BR Gavai
- Status: Decided
- Verdict: for the Petitioner
*updated May 8, 2022
Background
a PIL filed by Dr.Jacaob Puliyel, challenging the vaccine mandates and seeking publication of the clinical trial and adverse events of vaccination. [1]
Dr. Jacob Puliyel, a former member of the National Technical Advisory Group of Immunization had approached the Apex Court assailing the constitutional validity of the vaccine mandates imposed by States, in particular, Delhi, Madhya Pradesh, Maharashtra and Tamil Nadu. He had sought the indulgence of the Court to issue directions to the concerned authorities to disclose the data pertaining to clinical trials of the COVID-19 vaccines administered to adults as well as children in India, as per the requirement of International Medical norms. The petitioner also impelled the Court to revamp the Adverse Events Following Immunization Reporting System which he alleged was opaque, flawed and unknown to the public at large. [1]
Petitioner Jacob Puliyal has said that even though the central government is saying that vaccination is optional, it is not mandatory, but in states like Delhi, Tamil Nadu, Maharashtra and Madhya Pradesh, it has become mandatory. [2]
lawyer Prashant Bhushan had said that when the central government has said on many occasions, in response to statements and RTI that vaccination is not compulsory but optional, then in many states to open a shop, enter a shop or establishment. Vaccination certificates are sought on occasions such as entry of employees and people working there, walking on the streets, entering an educational institution? The petitioner, in his petition, has also referred to the circulars issued by the Government of National Capital Territory of Delhi on October 8 last year, November 8 in Madhya Pradesh, November 27 in Maharashtra and November 18 in Tamil Nadu and clearly written guidelines in which vaccination should be done. Essential restrictions have been imposed. [2]
At the same time, the Central Government opposed the petition to give the data of the clinical trial of Corona vaccine and not to force the vaccine. In the Supreme Court, the central government had said that such petitions filed for the vested interest of some people can affect the vaccination process. Even an oral comment of the court can be harmful. [2]
The Center told the Supreme Court that till November 24, 2021, one billion 19 crore 38 lakh 44 thousand 741 doses of corona vaccine have been given. Out of these, 2116 cases of adverse event following immunization i.e. AEFI have been registered so far. A report of rapid review and analysis has been completed for 495 (463 Coveshield and 32 Covaxin). Another report of 1356 cases (1236 Covishield, 118 Covaxin and 2 Sputnik) of severe AEFI cases (including 495 cases already analyzed) has been submitted to NEGVAC. [2]
The remaining cases are under rapid review and analysis and will be completed soon. On behalf of the Central Government, Solicitor General Tushar Mehta had said that this petition should not be heard. This may increase hesitation for the vaccine. The country has come out of it with great difficulty. [2]
Justice Nageswara Rao had said that that is why we said that if you have some specific facts, then they should be heard. We also do not want that there should be any problem regarding vaccination. [2]
In fact, on 9 August 2021, the Supreme Court had issued a notice to the Central Government on the petition not to compel people to apply the vaccine and make the trial data public. However, the Supreme Court refused to impose an interim stay on forcing the vaccine to be administered. Justice L Nageswara Rao said that 50 crore people in the country have been vaccinated. The WHO has also said that vaccine hesitancy has done a lot of damage. [2]
Lawyer Prashant Bhushan had said that according to the sero report, 2/3 people have been infected with Covid. In such a situation, the anti body is more effective than the corona vaccine. Now a policy has been made that if the vaccine is not applied then one cannot travel. Many restrictions have been imposed. The government is not making clinical data public. Since the vaccine is voluntary, then if someone does not get the vaccine, then he should not be denied any facility. The petitioner’s lawyer Prashant Bhushan has filed an application asking that the clinical trial of the vaccine as well as the data regarding the adverse effect of the vaccine be made public. [2]
India made headlines last year when it refused to offer a liability shield to Pfizer or Moderna, unlike countries such as Canada — which still has a federal vaccine mandate and a ban on the unvaccinated for travel. As such, no contract was signed between India’s authorities and these vaccine manufacturers. Instead, India relied on their own domestically produced vaccines. [3]
Pfizer infamously wanted to hide their COVID trial-related data for seventy-five years but was forced by a court order to release it. As their data dumps are being made publicly available bit by bit, public outrage continues to grow. [3]
Significance
This case is the first in the Supreme Court to decide on the legality of Vaccine Mandates
Plaintiff’s Argument
Vaccine Mandates
The sheet anchor of Advocate, Mr. Prashant Bhushan’s argument against the vaccine mandates was that in the absence of clinical trial data people were restrained from providing informed consent and the same impinged on the right to self-determination protected under Article 21 of the Constitution of India, 1950. Relying on K Puttaswamy v. UOI (2017) 10 SCC 1 and Common Cause v. UOI (2018) 5 SCC1, he emphasised that informed consent is necessary for medical procedures and bodily integrity is an integral part of the right to privacy. The Court was apprised that though the Government of India had indicated that vaccines are to be administered voluntarily, the States have imposed mandated restricting movement, denying essential services and curbing the right to livelihood in derogation of Articles 19 and 21. Mr. Bhushan argued that when there exists scientific evidence to substantiate the claims that nature immunity is better than vaccine-immunity; vaccination does not prevent from getting infected or transmitting; vaccines are ineffective in preventing new variants; vaccines have serious adverse effects; long-effects of the vaccine are unknown, mandating vaccination is unconstitutional. [1]
“For any vaccine to be mandated, the public health rationale underlying such a policy must be based essentially on efficacy and safety of vaccination and prevention of transmission of the disease“, Mr. Bhushan submitted. [1]
He referred to the decision of the UK Parliamentary Committee; judgement of the High Court of New Zealand in Yardley v. Minister for Workplace Relations and Safety [2022] NZHC 291 and orders of Gujarat High Court and Meghalaya High Court sticking down vaccine mandates. [1]
Non-Disclosure of data
Mr. Bhushan submitted that the segregated data of clinical trials of vaccines must be disclosed through peer reviewed scientific journals. The disclosure would have a significant impact on determining the adverse effects of the vaccines. The significance of disclosure was asserted by placing reliance on the Nuremberg Code and Report Nos. 59 (2012) and 66 (2013) of the Parliamentary Standing Committee on Health and Family Welfare. [1]
He informed the Court that an RTI Application was filed enquiring whether the Subject Expert Committee had looked at the raw days and/or discussed it. Responding to the same, the Central Drugs Control Standard Organisation stated that the brief of interim clinical trial results along with Subject Expert Committee’s recommendations was publicly available on CDSCO website. Dissatisfied with the response, an appeal was filed and the First Appellate Authority refused to reveal any data stating that the manufacturers had refused to disclose data publicly. [1]
Adverse Effect Following Immunization Reporting System
Mr. Bhushan submitted that besides it being an opaque and flawed system, there was a lack of public awareness about the same. [1]
Children’s Vaccine Mandate
Citing articles published in scientific journals, Mr. Bhushan argued that the overall risk from COVID-19 for children being remarkably low, it is not reasonable to vaccinate them, that too, without providing an opportunity to the parents to provide informed consent [1]
Rebuttal Arguments of the petitioner
Mr. Bhushan contended that the non-disclosure of trial data is preventing independent experts from making their own determinations. He stressed upon the petitioner’s plea that disclosure would permit the independent experts to look into the veracity of the claims of the manufacturers. In this regard, he referred to a United States District Court judgment, wherein the regulatory body was directed to disclose all the information pertaining to the Pfizer vaccine. [1]
He submitted that even considering the Government’s submission on privacy of the patients who participated in the trials, it ought to have made available segregated data. He emphasised that the assertion, vaccines significantly reduce the risk of transmitting the disease, had to be established by the Government by adducing evidence. Mr. Bhushan argued that by merely stating there exists a robust system for granting approval, it cannot be taken outside the ambit of judicial scrutiny. Mr. Bhushan asserted that the information available on the website pertains only to recommendations made by the expert bodies, but does not indicate the material on the basis of which such recommendations were made. [1]
With respect to the adverse reporting system, he pointed out that only the vaccinator can report such effects; the public at large have no knowledge about the reporting system and only known adverse effects can be reported. [1]
Defendant’s Argument
Argument from State Government of the Union Government
Solicitor General, Mr. Tushar Mehta at the outset, had questioned the bona fides of the petitioner. He contended that by way of a Public Interest Litigation, the petitioner cannot seek raw data of the clinical trial of the COVID-19 vaccines, merely to satisfy his curiosity, nor can he sit in judgment of the wisdom of domain experts. He refuted the claim of serious adverse effects. According to the official record till 13.03.2022, 1,80,13,23,547 doses had been administered and 77314 people or 0.004% of the vaccinated population had been adversely affected. Refuting the submissions made by Mr. Bhushan, alleging irregularities in the vaccine approval process, he took the Court through the statutory framework and submitted that the same had been adhered to while granting approval. Referring to the Epidemic Diseases Act, 1897 and Disaster Management Act, 2005, he demonstrated the wide ambit of power entrusted upon the Central Government to take measures during a pandemic. [1]
Mr. Mehta vehemently opposed the claim of the petitioner that there was a lack of mechanism for addressing adverse effects from immunization. On the issue of disclosure of clinical trial data, it was asserted that the same was in the teeth of confidentiality provisions. It was highlighted that the Helsinki Declaration and the WHO statement relied upon by the petitioner to seek raw clinical trial data only refers to the obligation to disclose final results, findings and outcomes which have already been disclosed.
Addressing the issue of children’s vaccine mandate, it was argued that evidence provided by the petitioner is based on mRNA vaccine, whereas the vaccine being administered in India was inactivated virus vaccines. It was further pointed out that for pediatric vaccines there is a statutory regime in place, which is being strictly followed. [1]
Mr. Mehta referred to a catena of foreign judgments with respect to vaccination in general, and the vaccination during the COVID-19 pandemic in particular to indicate that individual liberty is not absolute and is subject to other factors, like legitimate aim; and the necessity to achieve that aim.
Moreover he argued that the vaccine mandate is a matter of policy; a matter of scientific adjudication and the scope of judicial review in policy matters, especially when the executive decision is based on expert opinion, is limited. [1]
Argument from State Government of Tamil Nadu
Appearing for the State of Tamil Nadu, its Additional Advocate General, Mr. Amit Anand Tiwari submitted that the State Government has exercised power under Tamil Nadu Public Health Act, 1939 and the Disaster Management Act, 2005 to mandate vaccination for accessing public spaces. The mandate was justified, broadly on three grounds : [1]
It prevents mutation
Unvaccinated people causes health risk and
Economic impact.
Argument from State Government of Maharashtra
Advocate, Mr. Rahul Chitnis, appearing for the State of Maharashtra, submitted that the Government has mandated vaccination to enter shops, malls etc., and also to avail public transportation, but the same would pass the test of proportionality as expounded by the Apex Court in Modern Dental College And Research Centre And Ors. v. State of Madhya Pradesh. [1]
Argument from State Government of Madhya Pradesh
The Counsel adopted the submissions made by the Solicitor General about the need to balance rights. It was also clarified that the Government did not intend to make vaccines mandatory to avail ration. On the contrary, the purpose of the notification was to encourage individuals to get vaccinated. [1]
Argument from the Vaccine Manufacturers
Senior Advocate, Mr. Guru Krishnakumar, appearing for Bharat Biotech, controverted Mr. Bhushan’s argument that Phase III Trial of the vaccine has not been published. Moreover, it was emphasised that WHO guidelines referred to by the petitioner do not mandate the disclosure of the primary data and only the analysis of the data. Reliance was placed on Section 8(1)(d) of the Right to Information Act which exempts the disclosure of information including commercial confidence, trade secrets or intellectual property, the disclosure of which would harm the competitive position of a third party. The Counsel appearing for SII also opposed the petitioner’s plea for disclosure. [1]
Relevant Prior Judgements/ Cases
decision of the UK Parliamentary Committee; judgement of the High Court of New Zealand in Yardley v. Minister for Workplace Relations and Safety [2022] NZHC 291 and orders of Gujarat High Court and Meghalaya High Court sticking down vaccine mandates. [1]
Decision
The Supreme Court on Monday held that no individual can be forced to get vaccinated and the right to bodily integrity of a person under Article 21 of the Constitution include the right to refuse vaccinate. [1]
The Court also held that the vaccine mandates imposed by various state governments and other authorities in the context of COVID-19 pandemic are “not proportionate. The Court held so as no substantial data has been produced on record to show that the risk of transmission of COVID-19 virus from the unvaccinated persons are higher than from vaccinated persons. [1]
The Government is entitled to impose restrictions on individual rights in public health interests, but the restrictions should meet the 3-fold requirement legality, need and proportionality laid down by the Supreme Court in the Puttaswamy judgment. [1]
“No data has been provided by the Union of India or States before us controverting the material placed by petitioner which indicates that the risk of tranmission by unvaccinated is at par with vaccinated.In light of this the vaccine mandates cannot be said to be proportionate till the infection rate remains low and new development of research emergence which justifies the mandate”, the Court stated. [1]
Therefore, the Court suggested that all authorities, including private institutions and educational institutions, should review the restrictions on the unvaccinated. The Court however clarified that this direction is confined to the present context of the COVID pandemic situation. It further clarified that it does not extend to any other directions on COVID-19 appropriate behaviour issued by the authorities. [1]
Union’s vaccine policy not unreasonable or arbitrary.
The Court also held that the policy of the Union Governemnt on COVID-19 vaccination policy is reasonable. It also held that the clinical trial data of the vaccines have been published in accordance with the relevant norms. The material provided by the Union of India does not support the conclusion that emergency use approval has been granted in haste. [1]
Publish reports on Adverse Events
The Court also directed the Union of India to publish reports on Adverse Events Following Immunisation (AEFI) from public and doctors on a publicly accessible system without compromising data of the individuals who are reporting the same. [1]
Vaccination for children approved
Regarding vaccine for children, the Court said that it is not possible for us to second guess the opinion of experts and the vaccination indeed follows the global standards and practices. [1]
“On pediatric vaccine, it is in tune with international standards. We direct the Union of India to make sure the key findings of the stages of trial already approved for children be made public at the earliest”, the Court said. [1]
The Court rejected the arguments against the maintainability of the writ petition. Though executive has wide latitude in policy matters, it does not bar the Courts from scrutinizing if the policy is beyond the pale of arbitrariness.
Aftermath
…More information is needed…
Further Research
Court Documents:
- Read the Court Ruling
In the news:
On Corona Cases
Media
…

source: …
References
Keyword
Adverse, Adverse Events Following Immunisation, AEFI, Bharat Biotech, Bhushan, constitution, Delhi, Disaster Management Act, Effects, Epidemic Diseases Act, India, informed consent, Madhya Pradesh, Maharashtra, Mehta, Nuremberg Code, Puliyel, Reporting, Reporting System, Side, Supreme Court, System, Tamil Nadu, Unconstitutional, Vaccine Mandate
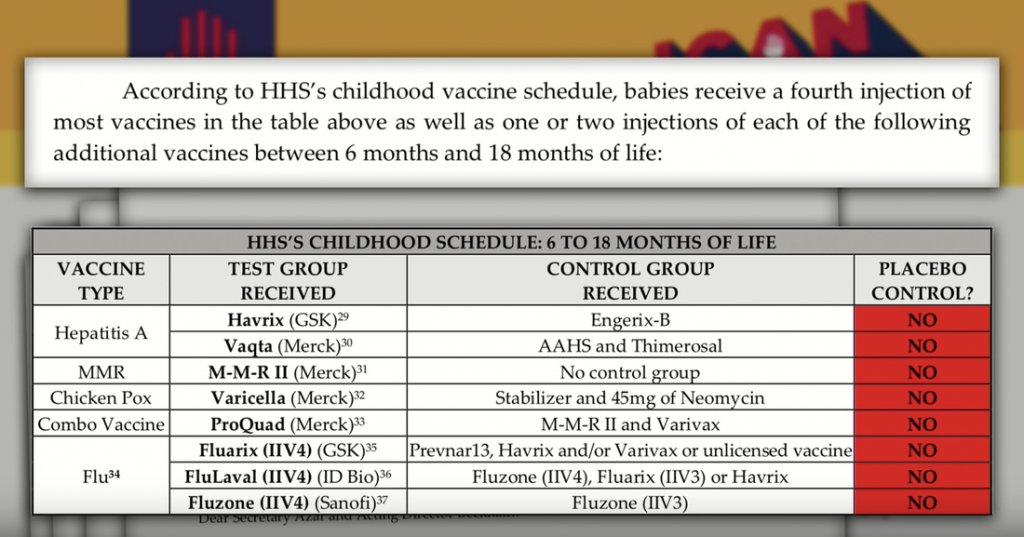
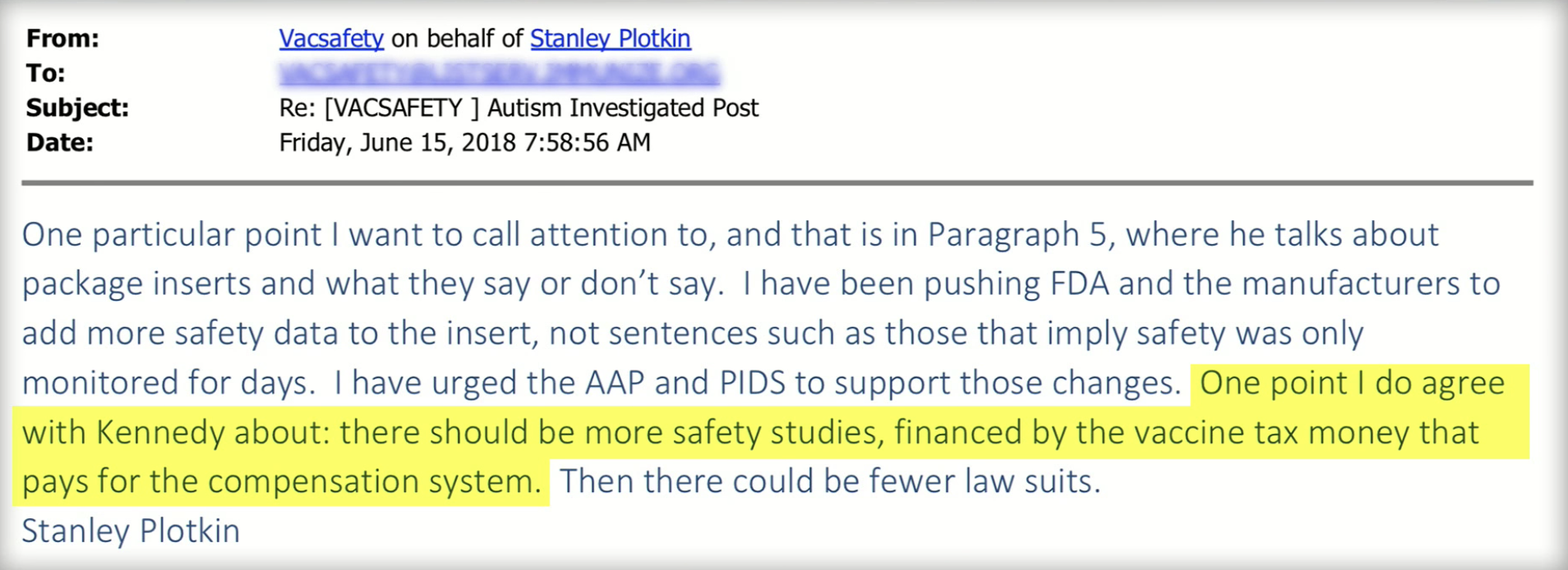
![[SOURCE] Page 14 ICAN Response December 31, 2018](http://coronacases.wiki/wp-content/uploads/2022/04/screen-shot-2019-01-27-at-3-44-49-pm_orig-1024x352.png)